Practice Essentials
Targeted temperature management (TTM), previously known as mild therapeutic hypothermia, in selected patients surviving out-of-hospital sudden cardiac arrest (OHCA) can significantly improve rates of long-term neurologically intact survival, [1] and it may prove to be one of the most important clinical advancements in the science of resuscitation.
Guidelines
The 2015 American Heart Association (AHA) guidelines on TTM can be summarized as follows [2] :
-
Induce hypothermia for unconscious adult patients with return of spontaneous circulation (ROSC) after OHCA when the initial rhythm was ventricular fibrillation (VF) or pulseless ventricular tachycardia (pVT) (class I, level of evidence: B-R)
-
Similar therapy may be beneficial for patients with non-VF/non-pVT (nonshockable) OHCA or with in-hospital arrest (class I, level of evidence: C-EO)
-
The temperature should be maintained between 32ºC and 36ºC (class I, level of evidence: B-R)
-
It is reasonable to maintain TTM for at least 24 hours (class IIa, level of evidence: C-EO)
-
Routine prehospital cooling of patients with ROSC with intravenous (IV) rapid infusion is not advised (class III: no benefit; level of evidence A)
-
It is reasonable to prevent fever in comatose patients after TTM (class IIb, level of evidence C-LD)
-
Hemodynamically stable patients with spontaneous mild hypothermia (>33°C) after resuscitation from cardiac arrest should not be actively rewarmed
Inclusion criteria
Patients who have been shown to benefit from induced hypothermia include the following (however, strict inclusion criteria vary by institution):
-
Intubated patients with treatment initiated within 6 hours after cardiac arrest (nonperfusing VT or VF)
-
Patients in a coma at the time of cooling
Exclusion criteria
Patients for whom hypothermia may theoretically carry increased risk include those with the following conditions:
-
Recent major surgery within 14 days - Possible risk for infection and bleeding
-
Systemic infection/sepsis - Small increase in risk of infection
-
Coma from other causes (drug intoxication, preexisting coma prior to arrest)
-
Known bleeding diathesis or with active ongoing bleeding - Hypothermia may impair the clotting system (however, patients may receive chemical thrombolysis, antiplatelet agents, or anticoagulants if deemed necessary in the treatment of the primary cardiac condition)
In addition, hypothermia is inappropriate in patients with a valid do not resuscitate order (DNR).
Cooling methods
Cooling methods include the following:
-
Surface cooling with ice packs
-
Surface cooling with blankets or surface heat-exchange device and ice
-
Surface cooling helmet
-
Internal cooling methods using catheter-based technologies
-
Internal cooling methods using infusion of cold fluids
Treatment protocols
The goals of treatment include achieving the target temperature as quickly as possible; in most cases, this can be reached within 3-4 hours of initiating cooling. Three phases of TTM include induction, maintenance, and rewarming. Rewarming can be begun 24 hours after the time of initiation of cooling, with avoidance of hyperthermia. [2]
External cooling with cooling blankets or surface heat-exchange device and ice
Before initiating cooling, confirm eligibility and gather materials.
-
Obtain 2 cooling blankets and cables (one machine) to “sandwich” the patient; each blanket should have a sheet covering it to protect the patient’s skin
-
Alternatively, place heat-exchange pads on the patient per the manufacturer’s recommendation
-
Pack the patient in ice (groin, chest, axillae, and sides of neck); use additional measures as needed to bring the patient to a temperature between 32°C and 36°C; avoid packing ice on top of the chest, which may impair chest wall motion
-
Monitor vital signs and oxygen saturation and place the patient on a continuous cardiac monitor, with particular attention to arrhythmia detection and hypotension
-
Once a temperature below the goal temperature is reached, remove ice bags and use the cooling blanket or heat-exchange device to maintain temperature between 32°C and 36°C [2]
Supportive therapy
-
A mean arterial pressure (MAP) goal of more than 80 mm Hg is preferred; hypertension is potentially additive to the neuroprotection of hypothermia
-
Norepinephrine can be used, starting at 0.01 mcg/kg/min and titrated to a MAP above 80 mm Hg
-
Practice standard neuroprotective strategies such as placing the head of the bed at 30° [5]
-
Obtain a 12-lead electrocardiogram (ECG) after ROSC to evaluate for the presence of ST-elevation (class I, level of evidence B) [2]
-
Monitor for dysrhythmia (most commonly bradycardia) associated with hypothermia
-
If life-threatening dysrhythmia arises and persists, or hemodynamic instability or bleeding develops, discontinue active cooling and rewarm the patient
-
During cooling, an ECG Osbourne or camel wave may be present; heart rate less than 40 bpm is common and is not a cause for concern in the absence of other evidence of hemodynamic instability
-
Check skin every 2-6 hours for thermal injury caused by cold blankets
-
Regularly check the patient’s temperature with a secondary temperature monitoring device when cooling
-
After TTM, fever should be avoided
-
Maintain oxygen saturation above 94%
-
Do not provide nutrition to the patient during the initiation, maintenance, or rewarming phases of the therapy
Controlled rewarming
Begin rewarming of the patient 24 hours after the initiation of cooling.
-
Rewarm slowly at a rate of 0.3-0.5°C every hour
-
Rewarming will take approximately 8-12 hours
-
Remove cooling blankets (and ice if still in use)
-
One method is to set the water temperature in the cooling device to 35°C and then increase the water temperature by 0.5°C every 1-2 hours until a stable core body temperature of 36°C has been reached for 1 hour
-
Maintain the paralytic agent and sedation until the patient’s temperature reaches 36°C; if infusing, discontinue the paralytic agent first; the sedation may be discontinued at the practitioner’s discretion
-
Monitor the patient for hypotension secondary to vasodilation related to rewarming
-
Discontinue potassium infusions
-
Avoid hyperthermia
See the image below.
Overview
The incidence of out-of-hospital sudden cardiac arrest (OHCA) in industrial countries is reported to be between 35.7 and 128.3 cases per 100,000, with a mean of 62 cases per year. [6] This translates into approximately 300,000 people in the United States and about the same number in Europe each year. Despite nearly 40 years of prehospital advanced life support, the survival rate of OHCA is very poor. [7] Less than half of victims who develop return of spontaneous circulation (ROSC) survive to leave the hospital alive, and the cause of death is anoxic brain injury in most patients with ROSC who die within one month of the cardiac arrest. Inducing mild therapeutic hypothermia in selected patients surviving OHCA has a major impact on long-term neurologically intact survival and may prove to be one of the most important clinical advancements in the science of resuscitation.
Some early great physicians, including Hippocrates, recognized the utility of hypothermia in attenuating injury. [8] The concept has experienced periodic reemergence in the medical literature, and recent studies of the modality date back mostly to the 1950s. In 1954, Hegnauer and D'Amato demonstrated decreased oxygen consumption in hypothermic dogs, [9] and the study by Benson et al of hypothermia after cardiac arrest in humans [10] demonstrated decreased mortality.
Until relatively recently, evidence for targeted temperature management (TTM) has lacked sufficient weight and the advisory panel support that thereby follows to propel it into common practice. Despite its 2005 inclusion in American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care, and 2003 advisory statements by the International Liaison Committee on Resuscitation (ILCOR) and the European Resuscitation Council (ERC), TTM is largely misunderstood and inconsistently applied. [11, 12, 13] The 2015 guidelines have modified the specific temperature range and duration of TTM on the basis of several more recent studies. [2]
A 2011 meta-analysis of randomized controlled trials found that TTM with conventional cooling methods improves both survival and neurologic outcomes at hospital discharge for patients who experienced cardiac arrest. [14]
In a retrospective cohort study covering 7 years that assessed the impact of TTM on early repolarization (ER) in survivors of cardiac arrest attributed to idiopathic ventricular fibrillation (ID-VF) compared with a control group who experienced coronary artery disease-related VF (CAD-VF), Williams et al found that hypothermia increased the prevalence and mean amplitude of ER in cardiac arrest survivors. [15] ER occurred in all survivors of ID-VF (100%) compared with over two third of survivors of CAD-VF (67%); TTM increased ER amplitude only in CAD-VF survivors. [15]
Two early studies demonstrated improved survival and neurological outcomes with induction of mild therapeutic hypothermia for comatose survivors of OHCA. The Hypothermia after Cardiac Arrest Study Group showed that, when applied to unconscious OHCA patients with ROSC (n=274), mild hypothermia (cooling to 32ºC-34ºC) provided significant improvement in functional recovery at hospital discharge (55% vs 39%; number needed to treat [NNT] = 6) and lower 6-month mortality rate when compared with patients who were not cooled (41% vs 55%) (NNT = 7). [16]
The NNT is very low and comparable to other important emergent treatments such as cardiac catheterization for acute coronary syndrome. [17] Bernard examined endpoint of survival to hospital discharge to home or a rehabilitation facility (good outcome) in 77 patients and demonstrated 49% in the hypothermia group compared with 26% in the normothermic group, finding an NNT of 4.5 for death and severe disability. [18]
After studying 133 comatose patients who experienced after OHCA and were treated with TTM, Kragholm et al found that one year later, most patients who were able to work prior to cardiac arrest were able to return to work. [19]
In a prospective, observational study that investigated the rate of good neurologic outcome based on the duration of resuscitation efforts in 86 OHCA) patients treated with TTM, Kim et al reported a median downtime of 18.5 minutes, with 33 patients (38.0%) having a good neurologic outcome. [20] Good neurologic outcomes were greatest when downtime was shortest: 62.5% at 10 minutes or less, 37% at 11-20 minutes, 25% at 21-30 minutes, and 21.7% at longer than 30 minutes. Interestingly, nearly one quarter of patients (22.9%) had a good neurologic outcome even with downtown longer than 20 minutes, a percentage that increased to 37.5% in patients with an initial shockable rhythm. [20]
A 2013 randomized control trial suggested no change in neurologic outcome and survival at 6 months in OHCA for 33ºC and 36ºC, followed by avoidance of fever for 72 hours. [21] Both groups in the study received some form of temperature management. The investigators found no difference in mortality and no difference in neurologic outcome between the groups. However, unlike the Bernard study, this trial included arrest other than VF/pVT OHCA. [21] Currently, advanced cardiac life support (ACLS) guidelines state that a temperature between 33ºC and 36ºC is recommended for at least 24 hours after achieving the target temperature. [2]
In a more recent systematic review and meta-analysis (2000-2016) that evaluated the effects of TTM on mortality and neurologic outcome, investigators found low-quality evidence supporting in-hospital initiation and maintenance of TTM at 32ºC-36ºC among adult OHCA survivors with an initial shockable rhythm for 18-24 hours. [22] There was no benefit of TTM for survivors of in-hospital cardiact arrest nor for those of OHCA with a nonshockable rhythm. In addition, there was no difference between endovascular and surface cooling TTM systems, and no benefit of adding feedback control to TTM systems. Moderate quality evidence also revealed no benefit for initiating prehospital TTM. [22]
In a multicenter retrospective study (2008-2013) that evaluated 1-year functional outcome (32ºC-34ºC) in 101 patients following intraoperative cardiac arrest (IOCA), Constant et al noted that less than one third (29.7%) received TTM. [23] Patients treated with TTM had an increased risk of infection but not with hemorrhage, arrhythmia, or metabolic/electrolyte disorders; TTM was not an independent prognosticator of 1-year favorable functional outcome after IOCA. [23]
Literature primarily evaluating different durations of TTM are lacking, as most studies maintain temperatures for at least 24 hours, with some longer (36 hours), followed by slow return to normothermia. Temperature sensitivity of the central nervous system following cardiac arrest can last as long as coma is present, and the duration of the TTM upper limit is unknown. [2]
TTM may also confer benefits to patients experiencing cardiac arrest in other clinical environments, [24] patients with hemorrhagic shock, [25] and patients with other forms of severe brain injury. [26, 27, 28, 29] Currently, at least 19 clinical trials are underway and focus principally on ROSC, traumatic brain injury, stroke, neonatal hypoxic-ischemic encephalopathy, and medical device safety. [30] Currently, no evidence supports using this modality for stroke. [31]
The process of selecting patients and initiating therapy is not complex. The ERC states that hypothermia is "safe and effective even if there is lack of experience." [32] The practice has been successfully applied in the academic tertiary care environment and in the community hospital setting, and it has been successfully implemented in the prehospital environment. [33, 34, 35, 36]
Implementation of hypothermia does require planning, education, and integration of multiple services within an institution. The basic framework for clinical protocols are well established, and institutions interested in establishing protocols may find a substantial amount of prior art in the literature (see hypothermia protocols).
Summary of 2015 AHA guidelines for CPR and emergency cardiovascular care regarding the use of hypothermia
Note the following [2] :
-
Induce hypothermia for unconscious adult patients with return of spontaneous circulation (ROSC) after OHCA when the initial rhythm was VF or pVT (class I, level of evidence: B-R)
-
Similar therapy may be beneficial for patients with non-VF/non-pVT (nonshockable) OHCA or with in-hospital arrest (class I, level of evidence: C-EO)
-
The temperature should be maintained between 32ºC and 36ºC (class I, level of evidence: B-R)
-
It is reasonable to maintain TTM for at least 24 hours (class IIa, level of evidence: C-EO)
-
Routine prehospital cooling of patients with ROSC with IV rapid infusion is not advised (class III: no benefit; level of evidence A)
-
It is reasonable to prevent fever in comatose patients after TTM (class IIb, level of evidence C-LD)
-
Hemodynamically stable patients with spontaneous mild hypothermia (>33°C) after resuscitation from cardiac arrest should not be actively rewarmed
Take home points
Note the following:
-
Have a plan and buy-in from necessary departments.
-
Experience with TTM is not needed to be successful.
-
Cool early (in the emergency department).
-
Use any cooling method.
-
Patients can continue to be cooled during percutaneous coronary intervention (PCI).
-
Use any pharmacologic agent necessary for primary cardiac condition (eg, aspirin, antiplatelet compounds, thrombolytics).
-
Treat patients as you would any critical care patient (tight glycemic control, vigilance for signs of infection, maintain perfusion, and use pressors if necessary).
-
Practice standard neuroprotective strategies such as placing the head of the bed at 30º and use seizure precautions.
-
Predict and be proactive regarding management of complications from ROSC and hypothermia, including shivering, fever, hypotension or hypertension, hyperglycemia, hypokalemia or hyperkalemia, bradycardia, and ongoing ischemia.
Pathophysiology
Cardiac arrest and return of spontaneous circulation (ROSC) is a case of whole-body ischemia and subsequent reperfusion injury. This injury mechanism along with pre-arrest comorbidities cause enormous biochemical, structural, and functional insults, which in a complex interrelated process leads to progressive cell destruction, multiorgan dysfunction, neuronal apoptosis, and death. [37] Many of these processes are temperature sensitive. Hypothermia has been shown to attenuate or ameliorate many deleterious temperature-sensitive mechanisms, thereby contributing to protection of the brain and heart.
The pathophysiologic mechanisms involved in hypothermia are incompletely understood but have been studied in cellular, animal, and human models.
The following actions are associated with hypothermia:
-
Reducing cerebral metabolism (approximately 6-8% per 1ºC) and demand
-
Reducing excitatory amino acids (glutamate release)
-
Attenuation and/or reversibility of ischemic depolarization of the central nervous system (CNS), leading to membrane stabilization, electrolyte redistribution, and normalization of intracellular water concentration and intracellular pH (stabilization of the blood-brain barrier)
-
Attenuation of oxygen free radical production and lipid peroxidation
-
Restoration of normal intracellular signaling mechanisms (including calcium modulation) and inhibition of deleterious signaling mechanisms, such as apoptotic signaling
-
Restoration of protein synthesis and gene expression
-
Inhibition of deleterious inflammatory products (ie, cytokines, interleukins, arachidonic acid cascade end products)
-
Attenuation of cerebrospinal fluid (CSF) platelet-activating factor (PAF)
-
Inhibition of cytoskeletal breakdown
In the heart, hypothermia may decrease the area of injury, promote epicardial reflow, decrease myocardial metabolic demand, and preserve intracellular high-energy phosphate stores. [38, 39, 40]
Patient Selection
Inclusion criteria
Patients who have been shown to benefit from induced hypothermia include the following; however, strict inclusion criteria vary by institution:
-
Intubated patients with treatment initiated within a 6-hour post cardiac arrest (nonperfusing ventricular tachycardia [VT] or ventricular fibrillation [VF]) time window
-
Patients able to maintain a systolic blood pressure above 90 mm Hg, with or without pressors, after cardiopulmonary resuscitation (CPR) [2, 3, 4] (This recommendation is primarily based on ensuring adequate end organ perfusion, as hypotension is associated with poor outcomes post arrest. Thus, clinicians should target adequate perfusion when beginning TTM.)
-
Those in a coma at the time of cooling. Coma is defined as not following commands. Brainstem reflexes and pathological/posturing movements are permissible. Patients with a Glasgow Coma Score (GCS) of 3 are eligible for hypothermia.
Exclusion criteria
Exclusion criteria are in part based on theoretical increases in risk. Many studies have reported increased but nonsignificant increases in risk. Patients for whom hypothermia may carry increased risk include those with the following conditions:
-
Recent major surgery within 14 days - Hypothermia may increase the risk of infection and bleeding.
-
Systemic infection/sepsis - Hypothermia may inhibit immune function and is associated with a small increase in risk of infection.
-
Coma from other causes (drug intoxication, preexisting coma prior to arrest)
-
Known bleeding diathesis or with active ongoing bleeding - Hypothermia may impair the clotting system. Check prothrombin time/partial thromboplastin time (PT/PTT), fibrinogen value, and D-dimer value at admission. (Note: Patients may receive chemical thrombolysis, antiplatelet agents, or anticoagulants if deemed necessary in the treatment of the primary cardiac condition.)
In addition, hypothermia is inappropriate for patients with a valid do not resuscitate order (DNR).
Induced hypothermia after pulseless electrical activity (PEA), asystole, or in-hospital arrest has not been fully studied. One large cohort study of cardiac arrest patients found that targeted temperature management (TTM) was not associated with good outcome in nonshockable patients. [41] Three observational studies have found no improvement in discharge neurologic outcome with TTM in patients with nonshockable rhythms, [18, 41, 42] although one study found reduced mortality at 6 months. [41]
Further investigation is needed, but TTM may be applied in these patients at the discretion of the treating practitioners. The practitioner should consider the most likely etiology of the cardiac arrest. For example, patients with PEA arrest due to septic shock may be poor candidates for hypothermia. Although their brain might benefit, the impairment to the immune system from hypothermia may be more significant.
Data from a study of prehospital hypothermia in 125 patients found that in those who had non-VF arrest—that is, PEA (n = 34), asystole (n = 39), or unknown rhythm (n = 1)—survival to hospital discharge was worse in the cooled group (6%) than in the noncooled group (20%). [36] This study was not intended or powered to detect differences in clinical outcome at discharge, but it raises concern regarding the use of hypothermia in patients with PEA or asystole and return of spontaneous circulation (ROSC) with hypothermia in the prehospital setting.
Induced hypothermia or TTM is not recommended for patients with an isolated respiratory arrest.
Treatment Protocols
Treatment goal
In most centers, the patient is actively cooled by using an induced hypothermia protocol for 24 hours to a goal temperature of 32ºC-36ºC. The goal is to achieve the target temperature as quickly as possible. In most cases, this can be achieved within 3-4 hours of initiating cooling. Rewarming is begun 24 hours after the time of initiation of cooling (ie, not from the time the target temperature is achieved). More evidence is needed to define the optimal duration of hypothermia treatment in humans. In animal models, effective hypothermia treatment can be less than 24 hours if initiated rapidly after return of spontaneous circulation (ROSC). In other animal models with long duration of cardiac arrest and delayed initiation of hypothermia, treatment for 48 hours is needed to achieve good neurological outcomes.
Preparation
Shivering, the body’s attempt at maintaining temperature homeostasis, is a concern when trying to achieve a hypothermic state. Shivering is uncomfortable, and it generates heat, interfering with the cooling process.
When using conventional surface cooling, sedation and chemical paralysis is usually necessary. Use of endovascular cooling can negate the need for paralysis (see the next section, Cooling Methods).
Cooling must be performed rapidly to achieve maximum effectiveness and should be instituted as early as possible. Most studies have found it necessary to use both cooling blankets and ice packs to achieve the temperature goal. Other methods such as ice lavage, cold saline infusion, and endovascular methods may be used to help achieve target temperature.
-
Do not actively rewarm patients who are spontaneously hypothermic.
-
When possible, hypothermia therapy for patients with out-of-hospital cardiac arrest should be initiated in the emergency department. Treatment can be continued while in the percutaneous coronary intervention (PCI) laboratory and in the intensive care unit (ICU).
-
Place an arterial line early for blood pressure monitoring as peripheral vasoconstriction will increase the difficulty of placing the line after the patient is cooled.
-
A continuous core temperature monitor should be used; this provides data to modulate cooling efforts and to avoid overcooling.
-
Esophageal, rectal, or bladder temperature is used to monitor the temperature.
-
Several cooling systems including liquid or gel heat transfer and endovascular systems incorporate a temperature probe (rectal, bladder, or attached to the endovascular coil system) that provides feedback to modulate the amount of cooling provided.
-
A pulmonary artery temperature probe may be used, if available.
-
A secondary temperature device should be used to monitor temperature as well. A bladder probe is only accurate when urine output is adequate; therefore, an alternative to the bladder temperature probe is required in the setting of oliguria. This alternative temperature probe can be any core temperature monitor (eg, esophageal).
Methods: Medication
Note the following:
-
Patient comfort and sedation: Follow agitation and pain guidelines for the institution. Parenteral narcotic analgesia can be provided with morphine or fentanyl; sedation can be maintained with agents such as midazolam or propofol.
-
Paralysis to prevent shivering: Buspirone (serotonin 1A partial agonist) and meperidine appear to lower the shivering threshold. [43] Continuous pharmacologic neuromuscular blockade is usually required. Use of an endovascular technique along with buspirone and surface warming may avert shivering without the need for paralysis. [44]
-
The train-of-four method of monitoring neuromuscular blockade may not function properly when the patient is cold. Many patients can have paralytic agents discontinued once the target core body temperature is achieved. If shivering is observed, then the neuromuscular blocking agent needs to be resumed.
-
Cold saline infusion can be given via a peripheral line or femoral venous catheter to assist in achieving goal temperature. The infusion is 30 mL/kg of 4ºC normal saline over 30 minutes. This is not to be used via a jugular or subclavian line because the safety via this method is not yet known.
A 2018 open-label multicenter trial that evaluated the the effect of a continuous infusion of a neuromuscular blockade in 81 comatose OHCA patients treated with TTM found no reduction in lactate levels nor improvement in survival or neurologic outcome at hospital discharge. [45] However, the investigators noted the study may have been underpowered to detect clinical differences, and thus further research is needed.
External cooling with cooling blankets or surface heat-exchange device and ice
Note the following:
-
Eligibility should be confirmed, and materials should be gathered.
-
Obtain 2 cooling blankets and cables (one machine) to “sandwich” the patient. Each blanket should have a sheet covering it to protect the patient’s skin.
-
Alternatively, place heat-exchange pads on the patient per the manufacturer’s recommendation.
-
Pack the patient in ice (groin, chest, axillae, and sides of neck); use additional measures as needed to bring the patient to a temperature between 32ºC and 34ºC. Avoid packing ice on top of the chest, which may impair chest wall motion.
-
Monitor vital signs and oxygen saturation and place the patient on a continuous cardiac monitor, with particular attention to arrhythmia detection and hypotension.
-
Once a temperature below 34ºC is reached, remove ice bags, and the cooling blanket or heat-exchange device is used to maintain temperature between 32ºC and 34ºC.
Supportive therapy
Note the following:
-
A mean arterial pressure (MAP) goal of more than 80 mm Hg is preferred from a cerebral perfusion standpoint. Hypertension is potentially additive to the neuroprotection of hypothermia. Norepinephrine can be used, beginning at 0.01 mcg/kg/min and titrated to a MAP greater than 80 mm Hg. The treating team should determine the MAP goal, balancing the cardiac safety with the theoretical advantage of higher cerebral perfusion pressures. Often, blood pressure remains elevated during hypothermia as a result of peripheral vasoconstriction. Hypotension is a concern during the warming phase.
-
Practice standard neuroprotective strategies such as placing the head of the bed at 30º. [5]
-
Monitor the patient for dysrhythmia (most commonly bradycardia) associated with hypothermia. If life-threatening dysrhythmia arises and persists, or hemodynamic instability or bleeding develops, then active cooling should be discontinued and the patient rewarmed. An electrographic (ECG) Osbourne or camel wave may be present when cooling. Heart rate less than 40 bpm is frequent and is not a cause for concern in the absence of other evidence of hemodynamic instability.
-
Hematologic testing recommendations include a complete blood cell (CBC) count, chemistry panel, troponin level, arterial blood gas (ABG) level, and partial thromboplastin time (PTT) at 0 hours. Hypothermia commonly causes hypokalemia, which may be exacerbated by insulin administration. Conversely, when patients are rewarmed, potassium exits cells, and hyperkalemia may occur. Repeat measurements of glucose, potassium, and ABG are needed every 6 hours. Unexplained increases in serum amylase and lipase levels have been observed during hypothermic therapy and appear to have limited clinical significance.
-
Potassium values less than 3.5 mEq/L should be treated while the patient is being cooled. Potassium administration should be stopped once rewarming begins.
-
Elevated serum glucose level is deleterious to the injured brain. Tight glycemic control should be maintained, although a specific range is not recommended by the American Heart Association (AHA). [2]
-
Normocarbia is advised, with the partial pressure of carbon dioxide (PaCO2) and end-tidal carbon dioxide (CO2) in the reference range (35-45 mm Hg for PaCO2; 30-40 mm Hg for end tidal CO2).
-
Avoid fever following targeted temperature management (TTM), as any elevated temperature is associated with worse neurologic outcome.
-
Avoid hypoxia, with administration of oxygen saturation above 94%. However, hyperoxia is also harmful.
-
Skin care should be checked every 2-6 hours for thermal injury caused by cold blankets.
-
Regularly check the patient’s temperature with a secondary temperature monitoring device when cooling.
-
Do not provide nutrition to the patient during the initiation, maintenance, or rewarming phases of the therapy.
Controlled rewarming
Rewarming of the patient is begun 24 hours after the initiation of cooling
The rewarming phase may be the most critical, as constricted peripheral vascular beds start to dilate. Peripheral hyperemia may cause hypotension. The literature recommends rewarming slowly at a temperature of 0.3ºC-0.5ºC every hour. Rewarming will take approximately 8-12 hours. Hyperthermia should be avoided.
Rewarming with any device or approach
-
Remove cooling blankets (and ice if still in use). The goal is to have the patient warm at about 0.3ºC-0.5ºC per hour up to a target of 36°C. One method is to set the water temperature in the cooling device to 35°C and then increase the water temperature by 0.5°C every 1-2 hours until a stable core body temperature of 36°C has been reached for 1 hour.
-
Maintain the paralytic agent and sedation until the patient’s temperature reaches 36°C. If infusing, discontinue the paralytic agent first. The sedation may be discontinued at the practitioner’s discretion.
-
Monitor the patient for hypotension secondary to vasodilatation related to rewarming.
-
Discontinue potassium infusions.
-
The goal after rewarming is normothermia (ie, avoidance of hyperthermia). [46]
Cooling Methods
Mild hypothermia is induced by using surface, internal, or a combination of cooling methods. Thermal heat-exchange cooling pads (eg, Arctic Sun) using water or gel may have superior rate of cooling (1.33°C/h for water and 1.04°C/h for gel) compared with cooling with 30 mL/kg iced saline plus ice packs (0.31°C/h). [47] Endovascular cooling may obviate the need for paralysis and provide very tight temperature control.
Surface cooling with ice packs
This method is inexpensive and represents an appropriate way to initiate cooling. However, it can be messy and is less than optimal in the rate of cooling and target temperature maintenance. Practically, ice packs are placed in anatomic areas that have large heat-exchange capability (the head, neck, axillae, and groin) and are replaced when the ice packs have substantially melted. In addition to ice packs, evaporative cooling with fans has been used. The average temperature drop using ice packs is moderately slow and highly variable and is reported to be 0.03ºC-0.98°C per hour.
Surface cooling with blankets or surface heat-exchange device and ice
Conventional surface cooling blankets are also suboptimal because of poor surface contact with the patient’s skin. However, a combination of water-circulating cooling device and ice packs are effective at rapidly cooling patients and are fair at maintaining target temperature. Many clinical studies have used this combination. The patient is sandwiched between 2 blankets or the cooling device's heat-exchange pads are placed and then ice packs are applied. When the target temperature is achieved, the ice packs are removed and the blanket or device is used to maintain the target temperature. Relatively newer medical devices, like those shown below, are available that have adhesive gel that facilitates heat exchange and are considerably more effective in the rate and maintenance of cooling.
 Targeted temperature management (therapeutic hypothermia). This is the Alsius CoolGard 3000. It is used in conjunction with the endovascular cooling catheter shown in the next image.
Targeted temperature management (therapeutic hypothermia). This is the Alsius CoolGard 3000. It is used in conjunction with the endovascular cooling catheter shown in the next image.
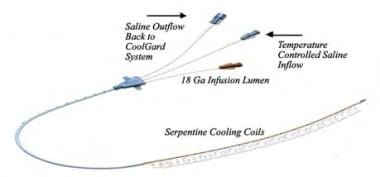 Targeted temperature management (therapeutic hypothermia). This is the Alsius Fortius 9.3 Fr endovascular cooling catheter.
Targeted temperature management (therapeutic hypothermia). This is the Alsius Fortius 9.3 Fr endovascular cooling catheter.
 Targeted temperature management (therapeutic hypothermia). This is the Arctic Sun heat-exchange device. It is used with the cooling pads as depicted in the next image.
Targeted temperature management (therapeutic hypothermia). This is the Arctic Sun heat-exchange device. It is used with the cooling pads as depicted in the next image.
Surface cooling helmet
Hachimi-Idrissi et al used a soft bonnetlike helmet cooling device that contained a solution of aqueous glycerol that facilitated heat exchange. [48] Although this method works, it may be slower than other methods.
Internal cooling methods using catheter-based technologies
Two internal cooling devices are currently available for use: the Celsius Control System and the Cool Line System. These technologies are also referred to as endovascular heat-exchange catheters. Heat exchange occurs between cooled saline that passes through the heat-exchange portion of the catheter (in a coil with large surface area for heat exchange) and the blood that flows over the outer surface of the catheter.
Endovascular cooling and rewarming is reported to be faster (1.46°C/h, [47] 1.59°C/h [44] ) and better at maintaining target temperature. These devices are generally placed in the femoral vein and are associated with a low rate of complications. Guluma et al reported endovascular cooling in conjunction with buspirone and surface warming allowed targeted temperature management (TTM) without shivering and avoided the need for chemical paralysis. [44] Extremely tight temperature control was also documented. [44] Thus, intravascular cooling allows:
-
Most rapid cooling
-
Tightest control of target temperature
-
Minimization of shivering and possible increase in the patient’s comfort as cutaneous warming is permitted
-
Opportunity to avoid need for chemical paralysis of the patient
Internal cooling methods using infusion of cold fluids
Many have studied the effect of cold fluid infusion for the induction of mild-to-moderate hypothermia in humans. The rates of induction are variable but otherwise considered to be rapid. Cold fluid infusion with concomitant use of cooling blankets has also been shown to be efficacious. Typical infusion volume is either 30 mL/kg or 2 L of fluid using either normal saline or lactated Ringer solution. In the several studies that have investigated cold fluid infusion, there has not been an association with increased venous pressure, left atrial filling pressures, pulmonary pressures, pulmonary edema, cardiac arrhythmia, or other major complications. [35, 47, 49, 50, 51, 52, 53]
Alternative Indications
Many indications for mild, moderate, and profound therapeutic hypothermia are currently being experimentally and clinically evaluated. Preliminary data, prospective studies, and meta-analyses suggest the indications for targeted temperature management (TTM) may broaden considerably in the future. TTM may be considered for any patient with severe brain injury without evidence of hemorrhage or elevated risk of cerebral hemorrhage at the discretion of the treating practitioner. However, currently, no expert consensus or advisory panel supports the broad application of hypothermia outside its current indication for comatose patients after return of spontaneous circulation (ROSC) due to ventricular fibrillation (VF) or ventricular tachycardia (VT).
Traumatic brain injury
TTM has been shown to be effective in traumatic brain injury (TBI) with high intracranial pressure (ICP) in adults. [54, 55] No benefit exists for patients with TBI with normal ICP. [27] Moderate therapeutic hypothermia adds no benefit and may increase incidence of hypothermia-related complications. [56, 57] Compared with short-term hypothermia (2-3 d), long-term hypothermia (5 d) significantly improves the outcome of patients with severe TBI with cerebral contusion and elevated ICP without causing significant complications.
Some research suggests that all patients with TBI may benefit from hypothermia (not just patients with elevated ICP) and that the level of prehospital hypothermia predicts efficacy. However, results from the National Acute Brain Injury Study: Hypothermia II (NABIS:H II), comprising data from 232 patients, showed that early induction of hypothermia was not effective as a primary neuroprotective strategy in adults aged 18-45 years with severe TBI. [58]
A 2008 literature review by Saxena et al found no evidence that modest hypothermia (35ºC-37.5ºC) in the first week after TBI resulted in improved outcomes, citing a lack of randomized controlled trials of post-TBI modest cooling therapies. [59] A more recent review by the same investigators in 2014 reiterated the lack of, and need for, randomized trials designed to evaluate patient outcomes with these interventions. [60]
Hypothermia in children can be considered an optional therapy for refractory intracranial hypertension but, to date, should not be regarded as standard of care. Studies have shown that hypothermia is safe and may be effective in selected pediatric patients with TBI; however, pediatric expert consensus requires more prospective clinical trials to be conducted. Mild therapeutic hypothermia may lower the seizure threshold and cause pulmonary hypertension in approximately 30% of pediatric patients treated with hypothermia. Complications can be predicted and safely treated in the intensive care unit (ICU) environment. [61, 62, 63]
In a 2018 post hoc analysis of a prospective multicenter trial (Brain Hypothermia [B-HYPO Study]), investigators found that after severe TBI, a mild decrease in heart rate during the early phase of TTM after tachycardia on admission is associated with unfavorable neurologic outcomes. [64]
Acute stroke
Considerable experimental evidence and clinical experience demonstrate that hypothermia reduces the volume of infarct and may preserve and restore the at-risk neurons in the penumbra in focal ischemic brain injury. [65, 66] Strong evidence suggests that hyperthermia (fever) is correlated with poor clinical outcomes. [67] Case-control studies and ongoing clinical trials in humans continue to evaluate the role of hypothermia in acute stroke. However, to date, large, prospective randomized human clinical trials to support the widespread adoption of hypothermia in acute stroke have not been completed. [66, 68]
Preliminary results from the prospective single-arm, open-label ReCCLAIM (Re perfusion and C ooling in C erebral A cute Ischem ia) study indicate that hypothermia (target temperature of 33ºC) can be safely used following intra-arterial reperfusion therapy (IAT) in patients with large pretreatment core infarcts. [69] Phase 2 of this trial will randomize patients with acute ischemic stroke to hypothermia or normothermia.
A 2016 pilot study of endovascular hypothermia with cold isotonic saline infusion (4ºC) in 26 patients with acute ischemic stroke demonstrated 100% technical success and no reports of obvious complications associated with the intra-arterial hypothermia. [70]
Pediatric cardiac arrest and neonatal hypoxic-ischemic encephalopathy
The International Liaison Committee on Resuscitation (ILCOR) advocates the use of TTM following perinatal asphyxia-related cardiac arrest in term newborns. [11, 71]
Traumatic spinal cord injury
Several review articles mention the use of hypothermia in patients experiencing traumatic spinal cord injury but, currently, no large case series assess the value of this intervention in these individuals.
Controversial Areas and Adverse Effects
Prehospital cooling
Prehospital initiation of cooling was originally thought to potentially improve outcomes, as earlier provision would benefit patients. However, several trials have compared cold intravenous fluids to no cooling during transport. To date, no cooling maneuvers begun in the prehospital period have improved neurologic recovery or mortality. [1, 36, 72, 73, 74, 75] One study suggested increased pulmonary edema and repeat cardiac arrest with 2 L of cold intravenous fluids. [75]
Cooling variables
It is likely that the variables of timing of the initiation of cooling, cooling technique, rate, depth, and length of cooling and rewarming all have some effect on mortality and morbidity. However, at this time, these variables are not well studied and are the focus of several experimental and clinical trials. Furthermore, these variables will most likely have different levels of importance on the basis of clinical indications.
Adverse effects
Hypothermia is associated with a number of adverse effects and complications. The main adverse effects reported are shivering, cardiac arrhythmia, sepsis, coagulopathy, and electrolytes and metabolic disturbances. [76, 77] Cold diuresis and hypovolemia may result. However, many large clinical trials and meta-analyses have reported these adverse effects to be infrequent. A trend toward increased incidence of sepsis has been noted.
Questions & Answers
Overview
What is the efficacy of targeted temperature management (TTM)?
Which conditions may benefit from treatment with targeted temperature management (TTM)?
What are the AHA guidelines for targeted temperature management (TTM)?
What is targeted temperature management (TTM)?
What are the AHA guidelines for targeted temperature management (TTM)?
When is targeted temperature management (TTM) indicated?
What are the exclusion criteria for targeted temperature management (TTM)?
Which cooling methods are used to achieve targeted temperature management (TTM)?
What are the stages of targeted temperature management (TTM)?
How is targeted temperature management (TTM) induced?
What is included in supportive therapy during targeted temperature management (TTM)?
How is controlled rewarming performed in targeted temperature management (TTM)?
What is the pathophysiology of targeted temperature management (TTM)?
What are the inclusion criteria for targeted temperature management (TTM)?
What are the exclusion criteria for targeted temperature management (TTM)?
When is targeted temperature management (TTM) contraindicated?
What is the treatment goal for targeted temperature management (TTM)?
How is shivering managed during targeted temperature management (TTM)?
Which cooling procedures are used in targeted temperature management (TTM)?
What is the role of medications in targeted temperature management (TTM)?
How is external cooling performed in targeted temperature management (TTM)?
What is included in supportive therapy during targeted temperature management (TTM)?
What are the protocols for rewarming in targeted temperature management (TTM)?
Which techniques are used to induce mild hypothermia in targeted temperature management (TTM)?
What is the role of ice packs in targeted temperature management (TTM)?
What is the role of surface heat-exchange devices in targeted temperature management (TTM)?
What is the role of a surface cooling helmet in targeted temperature management (TTM)?
Which internal cooling methods are used in targeted temperature management (TTM)?
What are benefits of endovascular cooling in targeted temperature management (TTM)?
What is the efficacy of internal cooling methods in targeted temperature management (TTM)?
Which indications for targeted temperature management (TTM) are under investigation?
What is the role of targeted temperature management (TTM) in patients with traumatic brain injury?
What is the role of targeted temperature management (TTM) in patients with acute stroke?
What is the role of targeted temperature management (TTM) in pediatric cardiac arrest?
What is the role of prehospital cooling in targeted temperature management (TTM)?
Which cooling variables affect the mortality and morbidity of targeted temperature management (TTM)?
What are the possible adverse effects of targeted temperature management (TTM)?
-
Targeted temperature management (therapeutic hypothermia). This is the Alsius CoolGard 3000. It is used in conjunction with the endovascular cooling catheter shown in the next image.
-
Targeted temperature management (therapeutic hypothermia). This is the Alsius Fortius 9.3 Fr endovascular cooling catheter.
-
Targeted temperature management (therapeutic hypothermia). This is the Arctic Sun heat-exchange device. It is used with the cooling pads as depicted in the next image.
-
Targeted temperature management (therapeutic hypothermia). Cooling pads.
-
Targeted temperature management (therapeutic hypothermia). Overview of neuronal apoptosis and approaches to inhibit this cell death cascade. Caspases play an important role as signal transducers (caspases 8, 9, 10) and as terminal executioners (caspases 3, 6, 7) in apoptosis. More recent neuroprotective therapeutic strategies involve enhanced activation of survival receptors (eg, via erythropoietin [EPO]). P = phosphate group, ROS = reactive oxygen species. Image and caption courtesy of Dr. Bernd Böttiger, Professor and Chairman of the Department of Anaesthesiology and Postoperative Intensive Care Medicine, University of Cologne.